Beyond Air, Inc. (XAIR) Stock: Orphan Drug Win Sparks Volatility Amid NeuroNOS Cancer Pipeline Hype
TLDR:
- Beyond Air soars on FDA nod, then dips as early-stage fears cool sentiment
- FDA boost lifts Beyond Air 49%, but preclinical status stirs caution
- NeuroNOS wins ODD for brain cancer drug; Beyond Air stock sees whiplash
- BA-101 earns FDA support, but no human trials yet spook investors
- Beyond Air’s glioblastoma hopes rise fast, fall hard on clinical delays
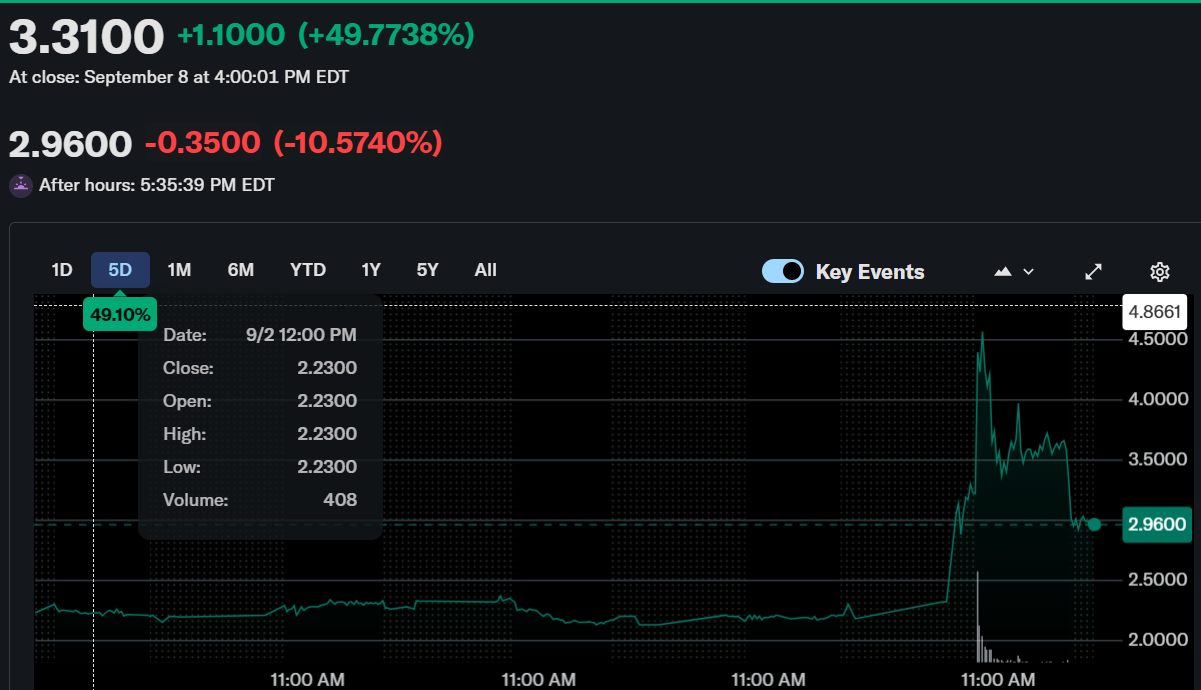
Beyond Air, Inc. (NASDAQ: XAIR) saw its stock surge 49.77% intraday on September 8, closing at $3.31. However, momentum reversed in after-hours trading as the stock declined 10.57%, ending at $2.96. This sharp fluctuation followed news of a key regulatory milestone achieved by its subsidiary, NeuroNOS.
Beyond Air, Inc. (NASDAQ: XAIR)
The U.S. FDA granted Orphan Drug Designation (ODD) to NeuroNOS’s investigational therapy, BA-101, for glioblastoma treatment. The designation delivers significant regulatory benefits, including tax credits, user fee waivers, and seven-year market exclusivity. These incentives strengthen the program’s commercial viability and reflect progress in advancing rare disease therapies.
The announcement highlights Beyond Air’s strategic push into neuro-oncology through its subsidiary’s pipeline. BA-101 targets glioblastoma using a nitric oxide (NO) modulation approach, positioning it as a novel treatment candidate. While promising, the compound remains in the pre-clinical phase, with first-in-human trials still on the horizon.
BA-101 Advances with Regulatory Backing
NeuroNOS secured ODD status as it aims to accelerate development of BA-101 for glioblastoma, a fast-progressing brain cancer. The therapy uses a nitric oxide inhibition mechanism, a novel scientific approach with emerging preclinical data. Although unproven in humans, the research indicates potential in modulating biological response to current treatments.
NeuroNOS receives federal support through waived FDA fees and development cost offsets via tax incentives. More critically, the seven-year U.S. market exclusivity strengthens commercial prospects if the drug obtains final FDA approval. This exclusivity acts independently from patent protections, offering a longer revenue window.
The preclinical stage of BA-101 means market readiness remains years away. NeuroNOS’s focus on rare diseases and neurological disorders supports a pipeline strategy driven by regulatory leverage. The glioblastoma designation marks its second ODD award, expanding its scope into oncology applications.
Glioblastoma Market Highlights Urgent Unmet Need
Glioblastoma is the most common malignant brain tumor in adults and offers limited treatment success. Standard-of-care includes surgery, radiation, and chemotherapy, yet median survival remains under 12 months. Long-term survival rates stay below 10%, reinforcing the need for innovation.
BA-101 seeks to address this unmet need by targeting a biological pathway with minimal therapeutic coverage. NeuroNOS claims published and unpublished data link nitric oxide imbalance to tumor progression and resistance. The company believes BA-101 can rebalance NO levels, potentially improving outcomes in this hard-to-treat cancer.
The glioblastoma treatment market remains small but heavily incentivized by regulatory policy. Affecting fewer than 200,000 U.S. patients, the condition qualifies under FDA criteria for rare disease development. As a result, biopharmaceutical firms like NeuroNOS stand to gain from streamlined development pathways.
The post Beyond Air, Inc. (XAIR) Stock: Orphan Drug Win Sparks Volatility Amid NeuroNOS Cancer Pipeline Hype appeared first on CoinCentral.
You May Also Like

Stellar Launches Community Fund v7.0 to Boost Ecosystem Growth

Why Vitalik Buterin believes Ethereum will regain ‘lost ground’ in 2026
